Cathodic Protection of Metals
 Cathodic protection is the electrochemical protection which is based on the application of current.
Cathodic protection is the electrochemical protection which is based on the application of current.
In the field of manufacturing, generally, one or two types of such protection are used.
The first assumes connecting a product (cathode) that needs to be protected to the external source of current. Supporting inert electrodes serve as the anode. A similar scheme of protection is used on the drilling platforms, welded metal substrates as well as for the protection of underground pipelines. It is important to mention that the cathodic protection is effective for eliminating not only the general corrosion but also various concrete kinds of it.
The second type of cathodic protection assumes the sacrificial or galvanic defense mechanisms. Therefore, the cathodic polarization of the metal product happens during its direct contact with more electronegative metal. In this case, a more electronegative metal serves as the anode.
Anode (zinc, magnesium or aluminum) begins to utterly collapse. The second method of cathodic protection is used for the metal construction with the low consumption of current covered with isolation.
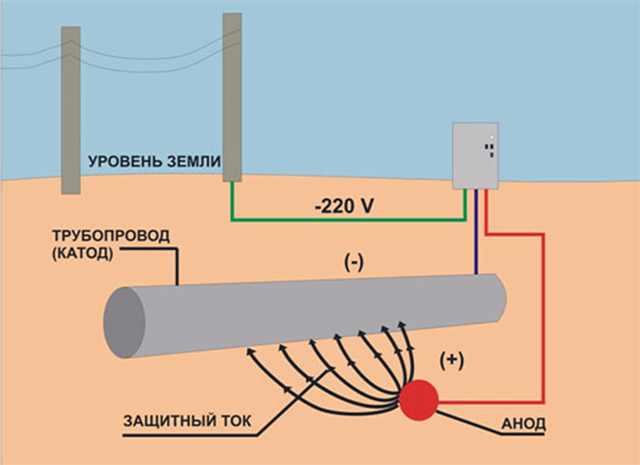
Such kind of protection is extremely effective. Thus, only one magnesium anode is able to protect up to eight kilometers of the coated pipes. That is why sacrificial protection is so popular and applicable in many countries around the world.




