Corrosion of the Underground Pipelines and their Protection
Corrosion of the underground pipelines is one of the major reasons of their depressurization due to the formation of cavities, cracks and breaks.
Corrosion of metal (i.e. its oxidation) is a transition from the free state into the chemically connected, ionic. Metal atoms, at the same time, lose their electrodes, while oxidants adopt them.
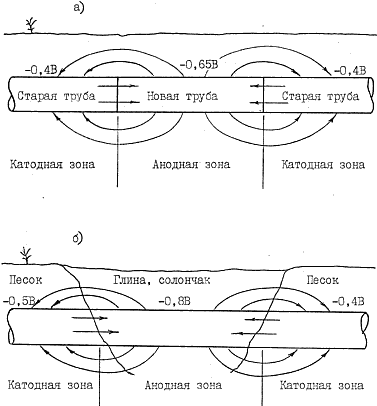
Рис 2.1
Due to the inhomogeneity of the metal pipe and soil heterogeneity (both physical characteristics and chemical composition), areas with different electrode potential appear on the underground pipeline, which causes the formation of the galvanic corrosion cells (pic. 2.1 and 2.2).
The most important types of corrosion are: surface corrosion (continuous over the entire surface), local corrosion in the form of shells, ulcer corrosion (pitting), slit corrosion, intergranular corrosion and fatigue corrosive cracking. The last two types are the most dangerous for the underground pipelines.
Surface corrosion only in rare cases can cause serious damages, while the ulcer corrosion causes the largest amount of damage.
КCorrosion-favorable situation in soil, where the metal pipeline is placed in, depends on the large number of factors regarding the soil and climatic conditions, track characteristics and operating conditions. Such factors are, as follows:
- soil moisture,
- chemical composition of the soil,
- the acidity of the soil electrolyte,
- soil structure,
- the temperature of the gas transported.
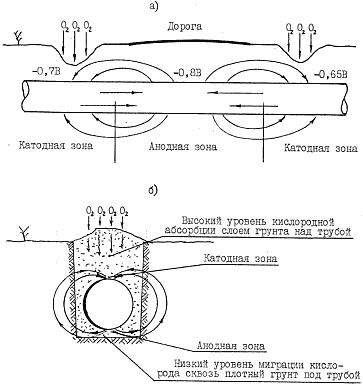
Рис. 2.2
The strongest negative development of the wandering currents in the ground, caused by the electrified rail transport of direct current is electro-corrosive destruction of the pipelines. The schematic demonstration of the development of the wandering currents and their influence on the pipeline is depicted in the picture 2.3.
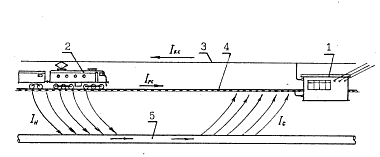
Pic. 2.3 Scheme of the development of the wandering currents on the railway with electric traction of the direct current.
1 - traction substation,
2 – load,
3 - contact network,
4 - running rail network,
5 – pipeline Iks – contact network current, Irs - running rail network current, In – current leaking on the pipeline, Is - current leaking from the pipeline.
Wandering current intensity and their influence on the underground pipelines depends on the following factors:
transitional ground-rail resistance;
the number of trains;
the distance between the traction substations;
the consumption of current by electric trains;
the number and the cross section of suction lines;
resistivity of the soil;
distance and location of the pipeline relatively to the way;
transitional and longitudinal resistance of the pipeline.
It is important to mention that the wandering currents in the cathodic zones serve as a means of protection of the product. Therefore, cathodic protection in such places could be performed without large financial expenses.
Methods of protection of the underground metal pipelines from corrosion are classified into passive and active.
Passive method of protection against corrosion offers creation impermeable barrier between metal pipeline and soil that surrounds it. This is achieved through the application of the special protective coat (bitumen, coal tar, polymer tape, epoxy resin and etc.).
In reality, it is impossible to gain full continuity of insulation coating. Different types of coating have different diffusive permeability, and thus, they provide different pipe insulation from the environment. During the process of construction and operation, cracks, dents and other defects can appear on the insulating coating. Perforating damage of the protective coating is the most dangerous one, where the soil corrosion actually flows.
Because the passive method does not provide a full protection of the pipeline from corrosion, it is necessary to apply active protection along with the passive one. Active method of protection is connected to the control of electrochemical processes, occurring at the metal pipe and soil electrolyte. Such type of protection is called comprehensive protection.
Active method of protection against corrosion is performed through the cathodic polarization, and is based on the lowering of the dissolution rate of the metal according to the shift of its corrosion potential into the range of more negative values than the natural potential.
In 1928, Robert Kun empirically established that the value of the potential of cathodic protection of steel is negative 0.85 Volts in relations to copper-sulphate reference electrode. Because the natural potential in the soil is approximately equal to -0.55…-0.6 Volts, in order to perform the process of the cathodic protection it is necessary to shift the corrosion potential by 0.25 ... 0.30 volts into the negative direction.
Applying electrical current between the surface of the metal pipe and the ground, it is necessary to decrease the potential in the defective places of the pipe insulation to the value lower than the protective potential criterion that is equal to 0.85 V. As a result, the corrosion rate will act to 10 microns per year, losing the practical value.
Cathodic protection of the pipelines can be performed using two methods:
- applying magnesium sacrificial anodes-protectors (galvanic method);
- applying external direct current sources, which is connected to the minus pipe, and plus - with the anode grounding (electrical method).
Galvanic method assumes that different metals in the electrolyte have different electrode potentials. If we form galvanic couple of two metals and place them in an electrolyte, the metal with more negative potential becomes anode and starts collapsing. Thus, it protects metal with less negative potential (pic. 2.4a).
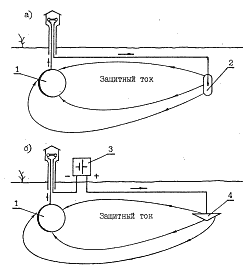
Pic. 2.4. Cathodic protection principle
a) using galvanic sacrificial anodes,
b) using polarization of the direct current.
1 – pipeline installed into the soil,
2 – galvanic sacrificial anode,
3 - constant current source,
4 - slightly soluble anode.
In practice, protectors made from magnesium, aluminum and zinc alloys are used as sacrificial galvanic anodes.
Application of the cathodic protection using protectors is effective only in the soil with low-impedance (up to 50 Ohm*m). In the soils with high-impedance such method does not provide all the necessary protection.
Cathodic protection performed by the external sources of the current, is more complex and time-consuming, but it hardly depends on the soil resistivity and has unlimited energy resource (pic. 2.4b).
Transducers of various designs powered by alternating current (AC) are usually used as direct current (DC) sources. Transducers help regulate protective current within the wide range, thus, providing pipeline protection in any conditions.
Overhead lines 0.4; 6; 10 kV and independent sources: diesel generators, thermogenerators, gas generators and etc. are used as power supply installations of cathodic protection.
Protective current applied on the pipeline by the manufacturer, creating a potential difference "pipe-to-earth" is unevenly distributed along the length of the pipeline. Therefore, the maximum absolute value of this difference is at the point of connection of the current source (point drainage). As the distance from this point increases, the potential difference "pipe-to-earth" is reduced. Excessive increase of the potential difference adversely affects the adhesion of the coating and can cause hydrogen absorption of metal pipes, which can become the reason for the hydrogen cracking. Decrease of the potential difference does not provide protection against corrosion and, in a certain range, can contribute to stress corrosion cracking.
Anodic protection is one of the methods of corrosion protection of metals in the aggressive chemical environments. It is based on the transferring metal from the active state to the inactive and keeling this state using external anode current. Cathodic protection high-alloy steels in the strong acids is impossible.
Unlike cathodic protection, anodic protection assumes only narrowly limited area of the protective potentials which provide protection against corrosion.




